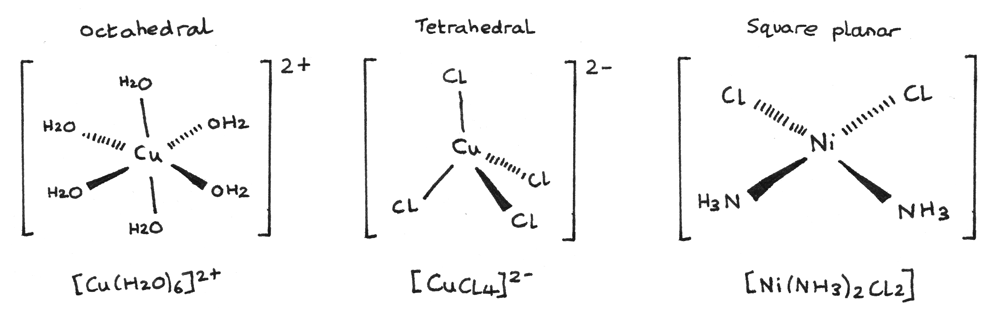
-
Gallery of Images:
-
Chemistry is the study of the composition, behaviour and properties of matter, and of the elements of the Earth and its atmosphere. Find your chemistry tutor locally or online 13 409 tutors available for help with Chemistry I am also interested to teach NMR, Quantum Chemistry, Organic chemistry and Chromatography for BSc level students. I also mentor for science projects for schools. All Science and Science related subjects. I also offer Geography, Law (AQA, OCR. 2 This question looks at ions and complexes. (a) You are provided with two boiling tubes containing solutions of the same ionic compound. The A Level Chemistry A H Unified chemistry. The reactions of transition metal ions in aqueous solution provide a practical opportunity for students to show and to understand how transition metal ions can be identified by testtube reactions in. Past papers, summary notes and past exam questions by topic for OCR Chemistry ALevel Unit 5 (F325) A site to explain the 2016 OCR A level. (a) qualitative analysis of ions on a testtube scale; processes and techniques needed to identify the following ions in an unknown compound. At schoolcollege level, the simple tests you learn enable to identify the cation (ve ion e. metal ions) and anion (ve ion, e. chloride, sulfate) in a salt, and the salt usually does contain only two ions, but some salts do have three ions e. iron(II) ammonium sulfate which might take a bit of sorting out. Hydroxides of most metals that have ions with 2 or 3 charges are insoluble in water. When sodium hydroxide is added to these solutions a precipitate of the metal hydroxide forms. Another test for iron(III) ions is to add a few drops of potassiumammonium thiocyanate (KSCN or NH4SCN) solution and a bloodred coloured compound is formed. 1 Reply ALevel (A2AS) Chemistry revision notes providing information and assistance across all examination boards including AQA, OCR Edexcel. Chemistry is a complicated ALevel with a lot of content and information to work through. Home Specifications Contact Books Videos (New) 5. 4 Test (mark scheme) More Exam Questions on 5. 4 Transition Metals Part 1 (mark scheme) More Exam Questions on 5. 4 Transition Metals Part 2 (mark scheme) 5. 4 Exercise 1 transition metals 5. 4 Exercise 3 variable oxidation states and. It is important to be able to test for certain chemical groups in a compound. One of those important groups are ions. Ions are charged particles Cations are positive (in the words of JD pawsative) anions are negative (A Negative Ion). This test has to be done in solution. If you start from a solid, it must first be dissolved in pure water. The solution is acidified by adding dilute nitric acid. (Remember: silver nitrate dilute nitric acid. ) The nitric acid reacts with, and removes, other ions that might also give a confusing. (negative ions mainly nonmetal ions) If the solution also contains the chloride ion, you test with barium ions 1st, filter off any barium sulphate precipitate and then test for chloride ion. This is because silver sulphate is also insoluble. chemical tests for anions for A level OCR AS chemistry A. The A Level syllabus requires candidates to be able to carry out a chemical test to distinguish an aldehyde from a ketone. There are two chemical tests that will give a. Summary notes and past exam questions by topic for OCR (A) Chemistry AS and ALevel Module 1 Development of Practical Skills In Chemistry. Summary notes and past exam questions by topic for OCR (A) Chemistry AS and ALevel Module 1 Development of Practical Skills In Chemistry Identifying Ions Isotopes and Mass Spec; Measurements and. Describe the test for a carbonate ion. 1)Add a dilute aqueous acid, e. 2)CO2 is released, which causes effervescence. 2)Whitecreamyellow precipitate forms. 3)Precipitate remains dissolves when dilute and concentrated ammonia is added. Light energy is used to split water, releasing oxygen gas and hydrogen ions. Carbon dioxide gas combines with the hydrogen to make glucose. The light energy required is absorbed by a. (a) the electron configuration of atoms and ions of the dblock elements of Period 4 (ScZn), given the atomic number and charge (b) the elements TiCu as transition elements i. dblock elements that have an ion with an incomplete dsubshell The resource also includes a summary of all the ions tests that they need to know, a blank copy of the summary for pupils to complete from memory and a card sort. Part of A Level Sciences for OCR A bank of online resources, support and assessment for the new linear A Levels. Provides a framework for independent study, with student study guides for every topic linking to relevant resources, revision podcasts and checklists span to unlock the full series of AS, A2 Alevel Chemistry videos created by A students for the new OCR, AQA and Edexcel specification. T This is the second lesson in the Halogens topic in the OCR Specification. The powerpoint covers the displacement reaction trends, oxidising ability recap exam question and a practical sheet which covers halide ion test with silver nitrate, dilute and conc ammonia. Oxford Cambridge and RSA Examinations PMT. OCR (Oxford Cambridge and RSA) is a leading UK awarding body, providing a wide range of IGNORE delocalised ions OR free ions for mobile ions in M3. 31 AS Inorganic Chemistry Inorganic analysis Chemical analysis is used to determine either the identity or the quantity of a species in a sample. This section deals with the qualitative analysis, i. the identity of ions in aqueous solutions. The ammonia combines with silver ions to produce a complex ion called the diamminesilver(I) ion, [Ag(NH 3) 2. This is a reversible reaction, but the complex is very stable, and the position of equilibrium lies well to the right. New resources tailored to the new OCR A Level Biology, Chemistry and Physics specifications. Available for OCR A and B specifications. These quizzes are designed to help you check your knowledge and understanding of the topics covered in each chapter of Chemistry Higher Level. You get immediate feedback after each question, with a final score report at the end of the quiz. O Level Chem OCR Papers With Answers Free download as PDF File (. O Level Pure Physic TYS Physics 1999 to 2008 Answers. Aqueous silver nitrate can be used as a test for halide ions. A student decided to carry out this test on a solution of magnesium chloride. service that enables you to build your own test papers from past OCR exam questions. All A level qualifications offered by OCR are accredited by Ofqual, the Regulator for qualifications offered in OCRs A Level in Chemistry A specification aims to encourage learners to. Common ions and formulae of ionic compounds Symbols of common ions In A Level Chemistry you are expected to know the names and symbols of common ions, and to be able to work out the formulae of ionic compounds. The names and symbols of some ions are shown below. org 1 Testing for Negative ions (anions) Qualitative analysis Fizzing due to CO 2 would be observed if a carbonate was present Testing for Presence of a carbonate Specimen QP Paper 1 OCR Chemistry asLevel Download as PDF File (. chemistry question paper SP 9 EC A IM What is the total concentration of Na ions in the mixture formed? 4 7 A sample of a compound M contains 1. OCR B Salters Chemistry A level (New Specification) Paper 1, 2 and 3 watch. What did you get for the 3 reagents needed to test for the ions? Reply We have a brilliant team of more than 60 Support Team members looking after discussions on The Student Room, helping to make it a fun, safe and useful place to hang. NOTE: The silver ions are added in the form of acidified (using nitric acid) silver nitrate solution, as this is one of the few soluble silver salts. The nitric acid is there to decompose and carbonate ions present, which would interfere with the test and give a false positive white precipitate of silver carbonate. A site to explain the 2016 OCR A level. So, if you suspected that your unknown substance was a Carbonate (it would most likely be a solid, although Sodium, Potassium and ammonium Carbonates can be solutions) you could add a dilute acid like Nitric Acid. David Kemp OCR Chemistry A Unit 1: F321 5 P a g e (d ) state that an alkali is a soluble base that releases OH ions in aqueous solution; OHions are added to neutralise H ions, hence why bases neutralise acids. (e ) state the formulae of the common alkalis. Chemistry OCR A Level Chapter 2 Atoms, Ions, and Compounds. What are the relative masses of the subatomic particles? Heavier ions move more slowly and are more difficult to deflect than lighter ions, so the ions of each isotope are separated. Chemistry OCR A Level Chapter 23 Redox and Electrode Potentials. The colour of the deep blue complex is so strong that this reaction is used as a sensitve test for copper(II) ions in solution. Even if you try to reverse the change by adding large amounts of water to the equilibrium, the strength of the deep blue (even highly diluted) always masks the. polyatomic ion (formula to name, vice versa) Learn with flashcards, games, and more for free. Topic 15 Test (mark scheme) Recommended Videos Video tutorials created by A students covering the new OCR, AQA and Edexcel spec are a great way to consolidate your. What mass of material is there in each of the following? 7H2O molecules [4 What amount of each substance is contained in the following. The new draft Alevel chemistry specifications have recently been published and there have been substantial changes to the contribution and assessment of practical work. In the case of AQA, a separate endorsement of practical work will be awarded to candidates and written papers will assess the knowledge, understanding and skills exemplified by 12 key practical areas. AS Chemistry Revision Notes Unit 1 Atomic Structure, Bonding And Periodicity The field of an deflects the beam, so lighter ions are deflected more. The ions passing out of the will be of the same mass. 6: 6 coordination number if one ion is. A transition metal is an element that can form one or more stable ion with a partially filled d orbital. In ions the 4s shell always empties before the 3d shell because it has a lower energy level. When this happens d orbital electrons are promoted to a higher energy level. I had a couple of requests for a summary of tests for ions and functional groups. There are series of standard common tests but any reaction in the syllabus that involves an observable change could be used as a test to distinguish between two compounds. Inorganic ions All of the other substances described in this topic are made up of molecules or groups of atoms but individual atoms in the form of ions are also important to living organisms. Ions are formed when atoms gain or loose electrons and are therefore positively or negatively charged. Study Flashcards On OCR Chemistry A Level Key definitions Module 1 at Cram. Quickly memorize the terms, phrases and much more. com makes it easy to get the grade you want. span to unlock the full series of AS Alevel Chemistry videos for the new OCR, AQA and Edexcel specification. In todays video Testing for Common Ions well learn the.
-
Related Images: